Imidacloprid 30.5% SC
Imidacloprid 30.5% SC
Product Details :-
- Chemical Name : 1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine
- Class : Neonicotinoid
- CAS Registry No : 138261-41-3
Structural Formula :-
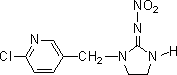
Specifications:-
| Description | The material shall consist of technical imidacloprid, complying with the requirements, dissolved in suitable solvents, together with any other necessary formulants. It shall be in the form of a clear or opalescent liquid, free from visible suspended matter and sediment, to be applied as a true solution of the active ingredient in water. |
| Pourability | Maximum “residue”: 4%. |
| Suspensibility | A minimum of 95% of the imidacloprid content found in suspension after 30min in CIPAC Standard Water D at 30 ± 2°C. |
| Wet sieve test | Maximum: 0.1% retained on a 75 μm test sieve |
| Persistent foam | Maximum: 40 ml after 1 min. |
| Stability at 0 ±1°C | After storage at 0 ± 2°C for 7 days, the formulation shall continue to comply with the clauses for: – Suspensibility – Wet sieve test |
| Stability at elevated temperature | After storage at 54 ± 2°C for 14 days, the determined average active ingredient content must not be lower than 97% relative to the determined mean content found before storage and the formulation shall continue to comply with the clauses for: – Pourability – Spontaneity of dispersion – Suspensibility – wet sieve test |

